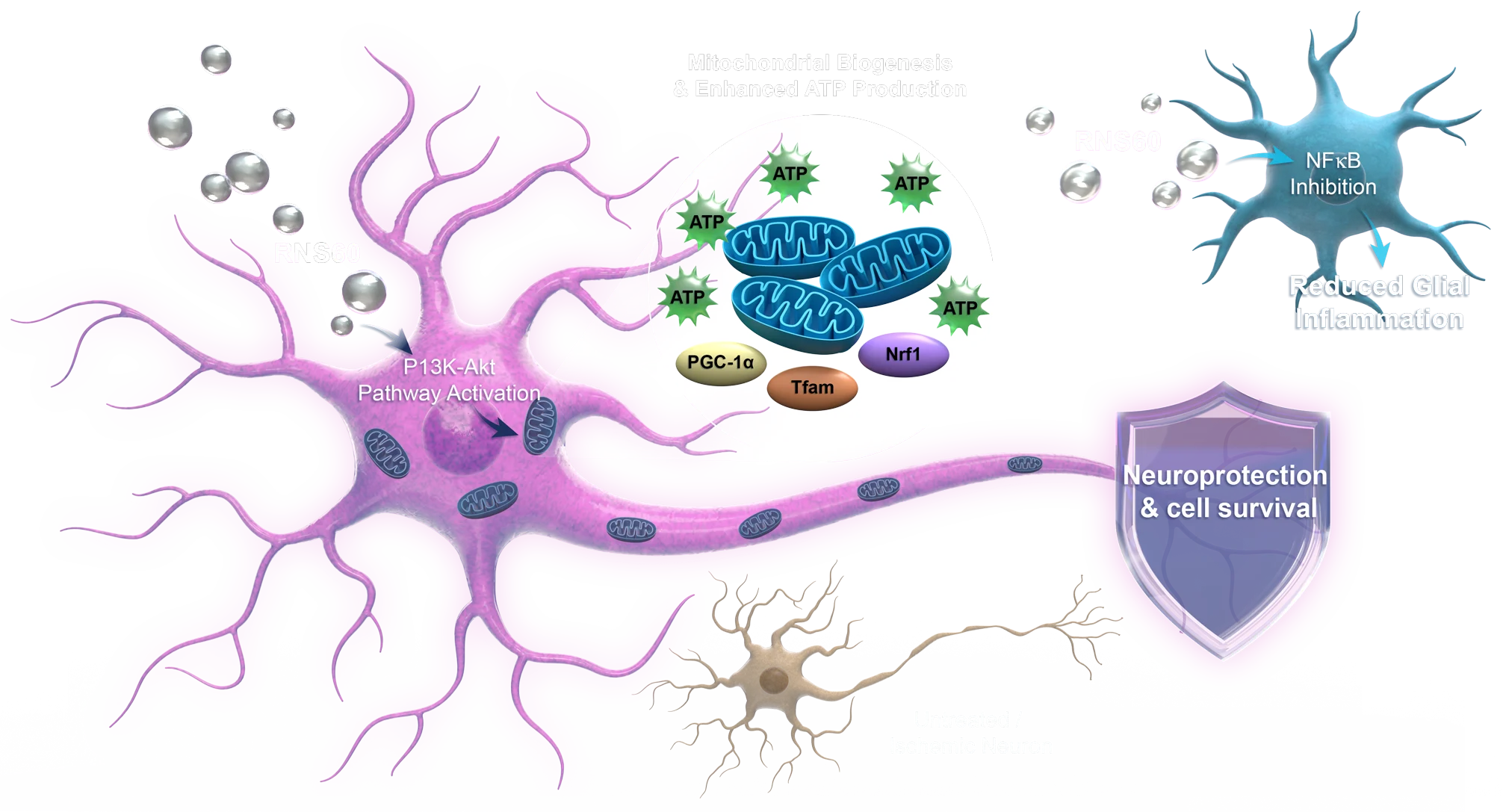
Our research continues to explore how protecting mitochondria can also help reduce brain loss and improve outcome in non-ischemic neuronal injury, as well as combat the progressive decline in neurodegenerative diseases and aging.
We have several discovery programs including:
- Traumatic Brain Injury
- Alzheimer’s Disease
- Parkinson’s Disease
- Age-Related Neurodegeneration